Pan‑tumor CGP solutions across tissue and liquid biopsy, providing enhanced coverage and sensitivity
CGP powered by TruSight™ Oncology 500 ctDNA v2 and TruSight™ Oncology 500 High-Throughput
TSO 500 HT and TSO 500 ctDNA v2
Unlock the complexities of every tumor
TSO 500 HT and TSO 500 ctDNA v2 provide enhanced coverage, sensitivity, and speed, supporting biopharma partners in confident, data-driven decision making. From biomarker discovery and target validation to resistance detection, clinical trial enrollment, longitudinal monitoring, and patient stratification – TSO 500 delivers the molecular clarity needed to advance precision oncology.
Our clinically and analytically validated CGP tests – spanning both tissue and liquid biopsy – work seamlessly to uncover comprehensive molecular insights across pan-solid tumor indications.
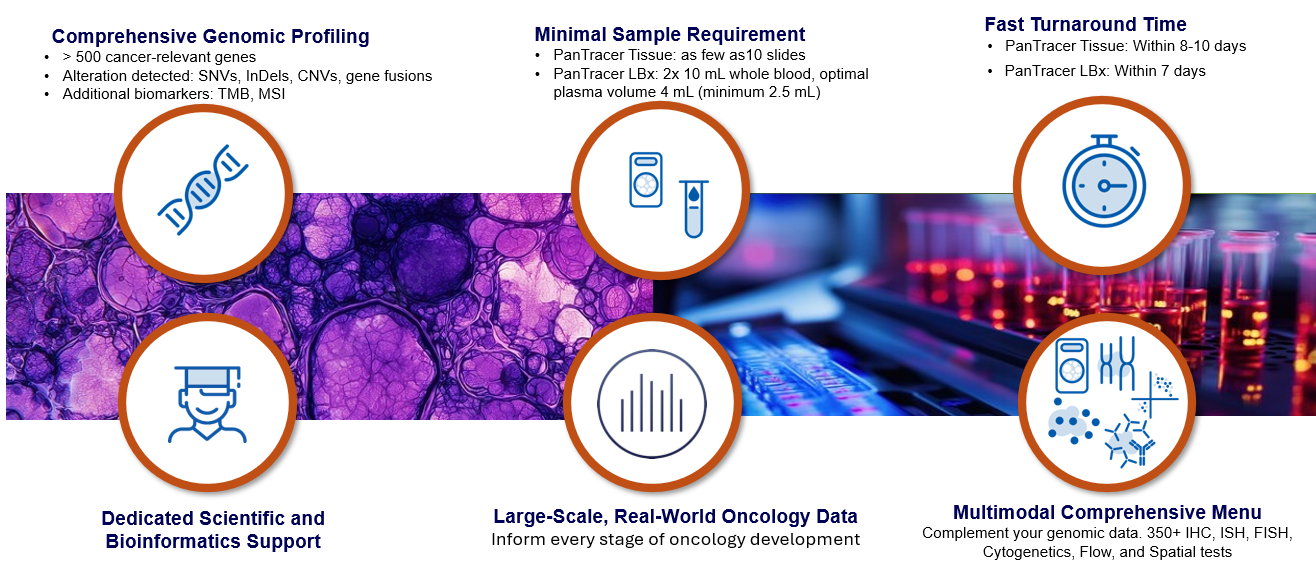
TSO 500 HT
TSO 500 ctDNA v2
Expertise You Can Trust. Resources You Can Act On.
 Explore our webinars, white papers, and other valuable resources
Explore our webinars, white papers, and other valuable resources