Colorectal cancer is the fourth most common type of cancer in the U.S.1
One in 24 people will be diagnosed with colorectal cancer in their lifetime.2
The NeoGenomics colorectal cancer solution supports the care continuum with tests that detect genomic alterations relevant to diagnosis, therapy selection, prognosis, and clinical trial options. Our portfolio includes tissue- and liquid-based testing methods such as next-generation sequencing (NGS), and immunohistochemistry (IHC) to help identify biomarkers like KRAS, NRAS, BRAF, MSI and MMR status.
Featured colorectal cancer solutions
Comprehensive testing for colorectal cancer patients
Over half of advanced-stage colorectal cancer patients have an actionable alteration, the most common being KRAS and NRAS mutations.3 Other relevant biomarkers include BRAF, MSI and MMR status, all of which can influence treatment choices. Genomic biomarker testing plays a crucial role in guiding precision medicine for patients with metastatic colorectal cancer with several FDA- approved targeted and immuno-therapy options now available for these molecular subtypes.
PanTracer Tissue covers key biomarkers recommended by clinical practice guidelines, using next-generation sequencing (NGS) and immunohistochemistry (IHC) where appropriate.
As the treatment landscape for colorectal cancer becomes increasingly complex, PanTracer simplifies treatment planning, removing guesswork and uncertainty so you can align patients with the most effective targeted therapies and clinical trials.
Guideline-driven biomarker detection for colorectal cancer4-7
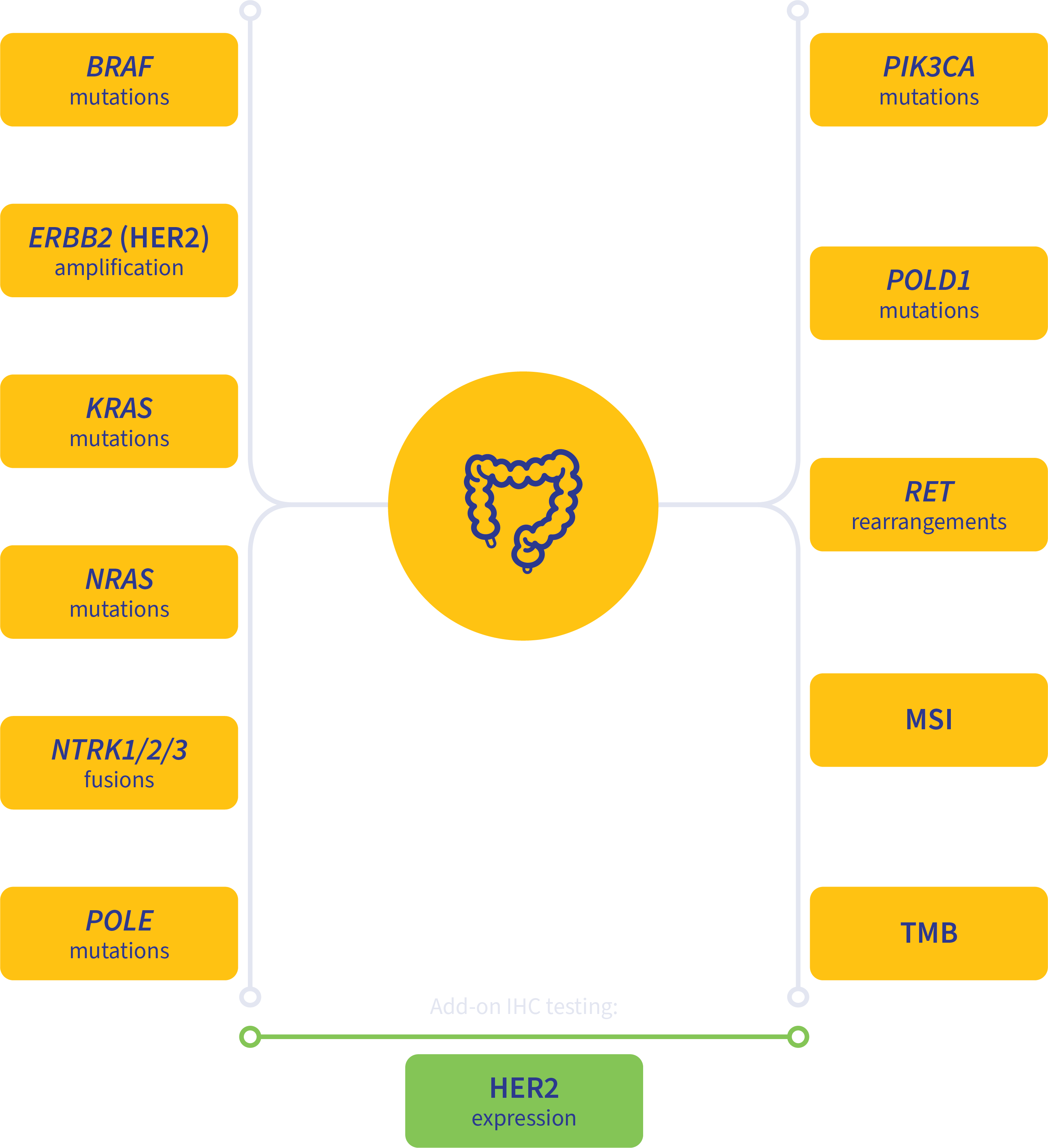
MSI, microsatellite instability.
Add-on testing for therapy selection
References
National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: colorectal cancer. Accessed December 23, 2024. https://seer.cancer.gov/statfacts/html/colorect.html
Colorectal Cancer Alliance. Colorectal cancer facts and statistics. Accessed December 23, 2024.
https://colorectalcancer.org/basics/facts-and-statistics
Saeed O, Lopez-Beltran A, Fisher KW, et al. RAS genes in colorectal carcinoma: pathogenesis, testing guidelines and treatment implications. J Clin Pathol.2019;72(2):135-139.
Yu IS, Aubin F, Goodwin R, et al. Tumor biomarker testing for metastatic colorectal cancer: a Canadian consensus practice guideline. Ther AdvMed Oncol. 2022;14:17588359221111705.
Hollasch M. Critical Recent NCCN Guideline Updates Affect Dozens of Malignancies. Oncol Live. 2024;25(16).
Cherri S, Oneda E, Zanotti L, Zaniboni A. Optimizing the first-line treatment for metastatic colorectal cancer. Front Oncol. 2023;13:1246716.
Chakravarty D, Johnson A, Sklar J, et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinicalopinion. J Clin Oncol. 2022;40(11):1231-1258.